| Combined injectable birth control | |
|---|---|
| Background | |
| Type | Hormonal |
| First use | By 1969 |
| Failure rates (first year) | |
| Perfect use | 0–0.2%[1] |
| Typical use | ? |
| Usage | |
| Duration effect | 1 month |
| User reminders | ? |
| Advantages and disadvantages | |
| STI protection | No |
| Benefits | Especially good if poor pill compliance |
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women. They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.
CICs are different from progestogen-only injectable contraceptives (POICs), such as depot medroxyprogesterone acetate (DMPA; brand names Depo-Provera, Depo-SubQ Provera 104) and norethisterone enantate (NETE; brand name Noristerat), which are not combined with an estrogen and are given once every two to three months instead of once a month.[2]
Hormonal contraception works primarily by preventing ovulation, but it may also thicken the cervical mucus inhibiting sperm penetration.[3][4][5] Hormonal contraceptives also have effects on the endometrium,[6][7] that theoretically could affect implantation.[8][9][10][11]
Medical uses
CICs are administered by intramuscular injection into the deltoid, gluteus maximus, or anterior thigh.[1] They are ideally administered every 28 to 30 days, though they have been demonstrated to be effective up to 33 days.[1]
Some CICs have been said to be used by transgender women as a means of feminizing hormone therapy as well.[12]
Available forms
| Composition | Dose | Vehicle | Brand Names | Availability |
|---|---|---|---|---|
| Estradiol valerate / Norethisterone enantate | 5 mg / 50 mg |
Oil solution | Multiple[a] | Approved in at least 36 countries |
| Estradiol cypionate / Medroxyprogesterone acetate | 5 mg / 25 mg |
Microcrystalline aqueous suspension | Multiple[b] | Approved in at least 18 countries |
| Estradiol enantate / Algestone acetophenidea | 10 mg / 150 mg |
Oil solution | Multiple[c] | Approved in at least 19 countries |
| 5 mg / 75 mg |
Oil solution | Anafertin†, Patector NF, Yectames | Approved at least 9 countries | |
| 10 mg / 120 mg |
Oil solution | Unalmes, Yectuna | Approved in at least 3 countries | |
| 10 mg / 75 mg |
Oil solution | Ova Repos† | Discontinued (firm was in Spain) | |
| Estradiol benzoate butyrate / Algestone acetophenide | 10 mg / 150 mg |
Oil solution? | Redimen, Soluna, Unijab, Unimens§ | Approved in Peru and Singapore |
| Estradiol valerate / Hydroxyprogesterone caproate | 5 mg / 250 mg |
Oil solution | Chinese Injectable No. 1 | Approved in China |
| Estradiol / Megestrol acetate | 3.5 mg / 25 mg |
Microcrystalline aqueous suspension | Chinese Injectable No. 2, Mego-E | Approved in China |
| Estradiol cypionate / Hydroxyprogesterone caproate | 5 mg / 250 mg |
Oil solution? | Sinbios† | Discontinued (firm was in Mexico) |
| Estradiol valerate / Estradiol benzoate / Hydroxyprogesterone caproate | 10 mg / 1 mg / 250 mg |
Oil solution? | Sin-Ol† | Discontinued (firm was in Mexico) |
Notes: All are given by intramuscular injection once a month. Footnotes: † = Discontinued. § = Never marketed. a = Unsorted brand names (doses unknown; for E2-EN/DHPA): Evitas† and Femineo†. Sources: [13][2][14][15][16][17][18][19][20][21][22][23]
| ||||
A variety of different CICs, generally containing a short-acting natural estradiol ester and a long-acting progestin ester, are available for clinical use.[24][15][2][16][13] Estrogens that are used include estradiol valerate, estradiol cypionate, estradiol enantate, estradiol benzoate butyrate, and estradiol, while progestins that are used include norethisterone enantate, medroxyprogesterone acetate, algestone acetophenide (dihydroxyprogesterone acetophenide), hydroxyprogesterone caproate, and megestrol acetate.[15][2][16][13] Estradiol benzoate has a duration that is too short for once-monthly CICs, and is not used in them.[25] Conversely, estradiol enantate is said to have a duration that is too long for once-monthly CICs, but is nonetheless used in them.[25]
Side effects
Side effects of CICs, besides menstrual bleeding changes, are minimal.[26] The most prominent side effects of CICs are menstrual irregularities during the first 3 to 6 months of use.[1] Dysmenorrhea has been reported in 30 to 65% of women.[26] Other side effects include breast tenderness/pain, headache, and libido changes.[26] Some fluid retention can occur, but weight gain is minimal.[26] Local injection site reactions have also been reported in 15 to 35% of women.[26]
Effects of CICs on coagulation and fibrinolysis are minimal and are not thought to be clinically relevant.[27] Conversely, combined oral contraceptive pills containing ethinylestradiol have considerable effects on coagulation and fibrinolysis.[27] The differences can be attributed to the lack of the first-pass effect with parenteral administration as well as structural and pharmacological differences between estradiol and ethinylestradiol.[28][29]
Pharmacology

CICs contain an estrogen and a progestin. The estrogen is generally a short-acting estradiol ester, which acts as a prodrug of estradiol.[24] Esters of estradiol are natural and bioidentical estrogens, and are believed to have more favorable effects on lipid metabolism, cardiovascular health, and hemostasis than synthetic estrogens such as ethinylestradiol.[30][31][32] The progestin is a long-acting progestogen ester, which may or may not act as a prodrug.[24] Progesterone derivatives including medroxyprogesterone acetate, algestone acetophenide (dihydroxyprogesterone acetophenide), hydroxyprogesterone caproate, and megestrol acetate are active themselves and are not prodrugs, whereas the testosterone derivative norethisterone enantate is a prodrug of norethisterone. Regardless of whether they are prodrugs or not, steroid esters form a depot and have an extended duration of action due to a depot effect when administered by intramuscular or subcutaneous injection.
Because CICs are administered parenterally, they bypass the first-pass effect in the liver and intestines that occurs with oral administration of estrogens.[24] However, is estimated that about 20% of an administered dose does still eventually pass through the liver.[24] Hence, these preparations are not completely liver-neutral.[24] Nonetheless, they have dramatically reduced hepatic effects relative to oral ethinylestradiol.[28] In addition, parenteral estradiol in general has about 4- or 5-fold reduced potency in the liver than oral estradiol.[28]
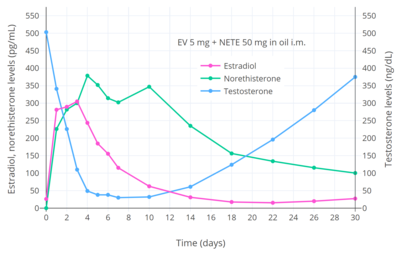
CICs have antigonadotropic effects via their estrogenic and progestogenic activity and inhibit fertility and suppress sex hormone levels. A single intramuscular injection of estradiol valerate/norethisterone enanthate (5 mg/50 mg) (Mesigyna) has been found to strongly suppress testosterone levels in men.[33] Testosterone levels decreased from a baseline of ~503 ng/dL to a trough of ~30 ng/dL (a 94% decrease) which occurred at day 7 post-injection.[33]
| Preparation | Form | Dose | Estradiol Cmax | Estradiol Tmax |
|---|---|---|---|---|
| EV/NETE | Oil solution | 5 mg/50 mg | 232–428 pg/mL | 2 days |
| EC/MPA | Aqueous suspension | 5 mg/25 mg | 184–736 pg/mL | 2–4 days |
| EEn/DHPA | Oil solution | 10 mg/150 mg | 314–317 pg/mL | 4.2–8.1 days |
| 5 mg/75 mg | 148 pg/mL | 6.5 days |
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
| Compound | Form | Dose for specific uses (mg)[c] | DOA[d] | |||
|---|---|---|---|---|---|---|
| TFD[e] | POICD[f] | CICD[g] | ||||
| Algestone acetophenide | Oil soln. | – | – | 75–150 | 14–32 d | |
| Gestonorone caproate | Oil soln. | 25–50 | – | – | 8–13 d | |
| Hydroxyprogest. acetate[h] | Aq. susp. | 350 | – | – | 9–16 d | |
| Hydroxyprogest. caproate | Oil soln. | 250–500[i] | – | 250–500 | 5–21 d | |
| Medroxyprog. acetate | Aq. susp. | 50–100 | 150 | 25 | 14–50+ d | |
| Megestrol acetate | Aq. susp. | – | – | 25 | >14 d | |
| Norethisterone enanthate | Oil soln. | 100–200 | 200 | 50 | 11–52 d | |
| Progesterone | Oil soln. | 200[i] | – | – | 2–6 d | |
| Aq. soln. | ? | – | – | 1–2 d | ||
| Aq. susp. | 50–200 | – | – | 7–14 d | ||
|
Notes and sources:
| ||||||
History
The first CIC to be studied was estradiol valerate/hydroxyprogesterone caproate (EV/OHPC) in 1963, and the second CIC to be studied was estradiol enantate/algestone acetophenide (E2-EN/DHPA) in 1964.[26][25] In 1967, E2-EN/DHPA was in the late stages of clinical development.[53][26] By 1969, the medication was available for medical use under the brand name Perlutal.[54] Within a few years, it was marketed under other brand names such as Topasel and Ova-Repos as well.[55][56][57][58] In addition, several other CICs had been introduced for medical use by 1972.[58] By 1976, two major CICs were in use: E2-EN/DHPA (brand names Perlutan, Topasel) in Spain and Latin America, and EV/OHPC (brand name Injectable No. 1) in China.[59] These CICs have been described as first-generation CICs.[59] Two second-generation CICs, estradiol cypionate/medroxyprogesterone acetate (EC/MPA; brand names Cyclofem and later Lunelle) and estradiol valerate/norethisterone enantate (EV/NETE; brand name Mesigyna), were introduced for clinical use in 1993.[60][14][15] On 5 October 2000, Pharmacia received FDA approval for Lunelle Monthly Contraceptive Injection.[1] In April 2003, Pharmacia was acquired by Pfizer (makers of depot medroxyprogesterone acetate).[citation needed] In October 2003, Lunelle was discontinued in the United States.[citation needed]
Society and culture
Availability

CICs are available in many countries throughout the world, including widely throughout Central and South America, in Mexico and the Caribbean, in China, in several Southeast Asian and African countries, and in Turkey.[21][22][23][13][2][14][15][16][17] They were also previously available in the United States, Portugal, and Spain, but have been discontinued in these countries.[22][23]
Research
Many other CICs have been studied but have not been approved or marketed for clinical use.[15][16][61][25][62][2]
The following are marketed CICs at different doses than those that are approved:
- Estradiol valerate 2.5 to 5 mg + norethisterone enantate 50 to 80 mg in an oil solution[15][16]
- Estradiol valerate 10 mg + hydroxyprogesterone caproate 500 mg in an oil solution[15]
- Estradiol cypionate 2.5 to 10 mg + medroxyprogesterone acetate 12.5 to 50 mg in a microcrystalline aqueous suspension[15][16]
- Estradiol enantate 5 to 50 mg + algestone acetophenide 75 to 200 mg in an oil solution[61][15]
The half-progestin-dose formulation of estradiol valerate/norethisterone enantate (5 mg / 25 mg) is also known as HRP-103 and the half-progestin-dose formulation of estradiol cypionate/medroxyprogesterone acetate (5 mg / 12.5 mg) is also known as HRP-113.[63]
The following are CICs that have never been marketed:
- Estradiol valerate 20 mg + medroxyprogesterone acetate 100 mg in a microcrystalline aqueous suspension[15][16]
- Estradiol undecylate 5 to 10 mg + norethisterone enantate 50 to 70 mg in an oil solution[25][15][62][64]
- Estradiol cypionate + norethisterone enantate[62][15]
- Estradiol valerate 10 mg + methenmadinone caproate 60 mg (Lutofollin)[25][62][64]
- Estradiol hexahydrobenzoate 5 mg (oil solution) + norgestrel 25 mg (aqueous suspension)[25][15][62][64][65]
- Estradiol cypionate 3.5 to 5 mg + megestrol acetate 25 mg in a microcrystalline aqueous suspension (marketed in China?)[25][15][62]
- Estradiol valerate 3 to 5 mg + chlormadinone caproate 80 mg in an oil solution[25][15][62]
- Estradiol valerate 5 mg + megestrol acetate 15 mg in an aqueous suspension of gelatin microspheres (50–80 μm)[25][16][62][32]
- Estradiol 5 mg + levonorgestrel 7 mg in an aqueous suspension of monolithic microspheres (80 μm) or in a macrocrystalline suspension (15 μm)[16][32]
- Estradiol cypionate 5 mg + levonorgestrel butanoate 7 mg in an aqueous suspension[16]
- Estradiol benzoate 5 to 10 mg + norethisterone enanthate 50 to 100 mg[62]
- Mestranol 1.0–1.2 mg + norethisterone 10–12 mg in a microcrystalline aqueous suspension of defined particle sizes (125–177 μm)[25][16][62][32]
- Ethinylestradiol + norethisterone[15]
- Estradiol 5 mg and progesterone 100 to 300 mg in an aqueous suspension of monolithic microspheres or in a macrocrystalline suspension[25][16][2][15][66][67][32]
- Polyestradiol phosphate 40 mg + medroxyprogesterone acetate 150 mg[68][69][70]
See also
- Special Programme on Human Reproduction
- Concept Foundation
- Extended cycle combined hormonal contraceptive
- Reproductive Health Supplies Coalition
- Estradiol-containing oral contraceptive
References
- ^ a b c d e "FDA Approves Combined Monthly Injectable Contraceptive". Contraception Report. 12 (3). 2001. Archived from the original on September 26, 2006.
- ^ a b c d e f g Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357. Archived from the original (PDF) on 2017-08-10. Retrieved 2016-08-24.
- ^ Tamara Callahan MD , Aaron Caughey MD , Blueprints Obstetrics and Gynecology, 2013
- ^ KD Tripathi , Essentials of Medical Pharmacology, 2013
- ^ Dc Dutta's Textbook of Obstetrics, 2014
- ^ K. A. Petrie, A. H. Torgal, C. L. Westhoff, Matched-pairs analysis of ovarian suppressionduring oral vs. vaginal hormonal contraceptive use, „Contraception” 2011, t. 84, p. e2-3
- ^ R. L. Birtch, O. A. Olatunbosum, R. A. Pierson, Ovarian follicular dynamics during conventional vs continuous oral contraceptive use, „Contraception” 2006, t. 73, p. 235. p. 239.
- ^ K. Bugge, K. S. Richter, J. Bromer, et al., Pregnancy rates following in vitro fertilization are reduced with a thin endometrium, but are unrelated to endometrial thickness above 10 millimeters,„Fertility and Sterility” 2004, t. 82, p. S199.
- ^ T. Fiumino, A. Kuwata, A. Teranischi et al., Significance of endometrium thickness to evaluate endometrial receptivity for embryos in natural cycle, „Fertility and Sterility” 2008, t. 90,p. S159.
- ^ K. S. Richter, K. R. Bugge, J. G. Bromer, Relationship between endometrial thickness and embryo implantation, based on 1. 294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos, „Fertility and Sterility” 2007, t. 87, p. 53.
- ^ Rivera R, Yacobson I, Grimes D (1999). "The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices". Am J Obstet Gynecol. 181 (5 Pt 1): 1263–9. doi:10.1016/S0002-9378(99)70120-1. PMID 10561657.
- ^ Don Kulick (12 January 2009). Travesti: Sex, Gender, and Culture among Brazilian Transgendered Prostitutes. University of Chicago Press. pp. 64–66. ISBN 978-0-226-46101-4.
- ^ a b c d IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0. Archived from the original (PDF) on 28 August 2021. Retrieved 18 September 2018.
- ^ a b c Pramilla Senanayake; Malcolm Potts (14 April 2008). Atlas of Contraception, Second Edition. CRC Press. pp. 50–. ISBN 978-0-203-34732-4.
- ^ a b c d e f g h i j k l m n o p q r Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- ^ a b c d e f g h i j k l m n o Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ^ a b IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 431–. ISBN 978-92-832-1291-1.
- ^ Klitsch M (1995). "Still waiting for the contraceptive revolution". Fam Plann Perspect. 27 (6): 246–53. doi:10.2307/2136177. JSTOR 2136177. PMID 8666089.
- ^ Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C (2013). "Combination injectable contraceptives for contraception". Cochrane Database Syst Rev. 2013 (3): CD004568. doi:10.1002/14651858.CD004568.pub3. PMC 6513542. PMID 23641480.
- ^ Harry W. Rudel; Fred A. Kinel (September 1972). "Oral Contraceptives. Human Fertility Studies and Side Effects". In M. Tausk (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 385–469. ISBN 978-0080168128. OCLC 278011135.
- ^ a b "International Drug Names from". Drugs.com. Retrieved 2022-04-30.
- ^ a b c Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- ^ a b c "Micromedex Products: Please Login".
- ^ a b c d e f V. Unzeitig; Rick H.W. van Lunsen (15 February 2000). Contraceptive Choices and Realities: Proceedings of the 5th Congress of the European Society of Contraception. CRC Press. pp. 133, 136. ISBN 978-1-85070-067-8.
- ^ a b c d e f g h i j k l Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ a b c d e f g Benagiano, G.; Primiero, F.M. (1983). "Long Acting Contraceptives Present Status". Drugs. 25 (6): 570–609. doi:10.2165/00003495-198325060-00003. ISSN 0012-6667. PMID 6223801. S2CID 45898359.
- ^ a b "Facts about once-a-month injectable contraceptives: memorandum from a WHO meeting". Bull. World Health Organ. 71 (6): 677–89. 1993. PMC 2393537. PMID 8313486.
- ^ a b c Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ von Schoultz, Bo; Carlström, Kjell; Collste, Lars; Eriksson, Ambjörn; Henriksson, Peter; Pousette, Åke; Stege, Reinhard (1989). "Estrogen therapy and liver function—metabolic effects of oral and parenteral administration". The Prostate. 14 (4): 389–395. doi:10.1002/pros.2990140410. ISSN 0270-4137. PMID 2664738. S2CID 21510744.
- ^ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 235–237, 261, 271. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- ^ Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 419–. ISBN 978-93-5090-575-3.
- ^ a b c d e Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ a b c d Valle Alvarez, Doris del Cisne (11 May 2011). Efecto de una Dosis de 50 mg de Enantato de Noretisterona y 5 mg de Valerato de Estradiol en los Niveles de Testosterona Total en Hombres Mexicanos Sanos [Effect of a Dose of 50 mg of Norethisterone Enanthate and 5 mg of Estradiol Valerate on Total Testosterone Levels in Healthy Mexican Men] (MSc). National Polytechnic Institute of Mexico.
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ^ Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 554–. ISBN 978-3-642-96158-8.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl K (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Ufer J (1969). The Principles and Practice of Hormone Therapy in Gynaecology and Obstetrics. de Gruyter. p. 49. ISBN 9783110006148.
17α-Hydroxyprogesterone caproate is a depot progestogen which is entirely free of side actions. The dose required to induce secretory changes in primed endometrium is about 250 mg. per menstrual cycle.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598, 601. ISBN 978-3-11-150424-7.
- ^ Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- ^ Henzl MR, Edwards JA (10 November 1999). "Pharmacology of Progestins: 17α-Hydroxyprogesterone Derivatives and Progestins of the First and Second Generation". In Sitruk-Ware R, Mishell DR (eds.). Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. pp. 101–132. ISBN 978-0-8247-8291-7.
- ^ Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. p. 114. ISBN 978-0-12-137250-7.
- ^ Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–385. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ Goebelsmann U (1986). "Pharmacokinetics of Contraceptive Steroids in Humans". In Gregoire AT, Blye RP (eds.). Contraceptive Steroids: Pharmacology and Safety. Springer Science & Business Media. pp. 67–111. doi:10.1007/978-1-4613-2241-2_4. ISBN 978-1-4613-2241-2.
- ^ Becker H, Düsterberg B, Klosterhalfen H (1980). "[Bioavailability of cyproterone acetate after oral and intramuscular application in men (author's transl)]" [Bioavailability of Cyproterone Acetate after Oral and Intramuscular Application in Men]. Urologia Internationalis. 35 (6): 381–385. doi:10.1159/000280353. PMID 6452729.
- ^ Moltz L, Haase F, Schwartz U, Hammerstein J (May 1983). "[Treatment of virilized women with intramuscular administration of cyproterone acetate]" [Efficacy of Intra muscularly Applied Cyproterone Acetate in Hyperandrogenism]. Geburtshilfe und Frauenheilkunde. 43 (5): 281–287. doi:10.1055/s-2008-1036893. PMID 6223851.
- ^ Wright JC, Burgess DJ (29 January 2012). Long Acting Injections and Implants. Springer Science & Business Media. pp. 114–. ISBN 978-1-4614-0554-2.
- ^ Chu YH, Li Q, Zhao ZF (April 1986). "Pharmacokinetics of megestrol acetate in women receiving IM injection of estradiol-megestrol long-acting injectable contraceptive". The Chinese Journal of Clinical Pharmacology.
The results showed that after injection the concentration of plasma MA increased rapidly. The meantime of peak plasma MA level was 3rd day, there was a linear relationship between log of plasma MA concentration and time (day) after administration in all subjects, elimination phase half-life t1/2β = 14.35 ± 9.1 days.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 429–. ISBN 978-3-642-73790-9.
- ^ Artini PG, Genazzani AR, Petraglia F (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 105–. ISBN 978-1-84214-071-0.
- ^ King TL, Brucker MC, Kriebs JM, Fahey JO (21 October 2013). Varney's Midwifery. Jones & Bartlett Publishers. pp. 495–. ISBN 978-1-284-02542-2.
- ^ Hecht-Lucari, G. (1967). Recientes Progresos de la Terapia Hormonal en Ginecología. Revista Colombiana de Obstetricia y Ginecología, 18(5), 307-319. 10.18597/rcog.2584 https://revista.fecolsog.org/index.php/rcog/article/view/2584
- ^ Hispano americano. Tiempo. May 1969. p. 46.
Entre los anovulatorios más usados están los siguientes: Prolestrín, Sequens, Anovlar, Sequentex, Orlex, Ginovlar, Enginón, Perlutal, Depo-proveda, Aconcén, Ovral, Retex, Lorophyn y otros menos solicitados.
- ^ Botella-Llusia, J. (1970). Les ovaires au cours de l'administration des sterpides anticonceptionnels. [The ovaries during administration of contraceptive steroids.] In: Netter, A. L'Inhibition de l'ovulation; Colloque de la Societe Nationale pour l'Etude de la Sterilite et de la Fecondite. (Inhibition of ovulation: Proceedings of the National Society for the Study of Sterility and Fertility.) Paris, Masson, 1970. p. 141-156
- ^ Universidad Complutense de Madrid (1971). Revista de la Universidad de Madrid. Prensa de la Universidad de Madrid. p. 11.
- ^ Liria, R. H. (1972). Anticoncepcionismo (Un problema de hoy, de ayer y de siempre). In Anales de medicina y cirugía (Vol. 52, No. 230, pp. 329-348). https://www.raco.cat/index.php/AnalesMedicina/article/download/99455/152590
- ^ a b Harry W. Rudel; Fred A. Kinel (September 1972). "Oral Contraceptives. Human Fertility Studies and Side Effects". In M. Tausk (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 385–469. ISBN 978-0080168128. OCLC 278011135.
- ^ a b J. Bringer; B. Hedon (15 September 1995). Fertility and Sterility: A Current Overview. CRC Press. pp. 47–. ISBN 978-1-85070-694-6.
- ^ d'Arcangues C (1993). "Once-a-month injectable contraceptives". World Health Forum. 14 (4): 439–40. PMID 8185807.
- ^ a b Koetsawang S (April 1994). "Once-a-month injectable contraceptives: efficacy and reasons for discontinuation". Contraception. 49 (4): 387–98. doi:10.1016/0010-7824(94)90034-5. PMID 8013221.
- ^ a b c d e f g h i j Mokhtar K. Toppozada (1983). "Monthly Injectable Contraceptives". In Alfredo Goldsmith; Mokhtar Toppozada (eds.). Long-Acting Contraception. pp. 93–103. OCLC 35018604.
- ^ Unlisted Drugs Pharm AID. Unlisted Drugs. 1993. p. 247. ISBN 978-0-913210-14-7.
- ^ a b c Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ^ de Souza, J. C.; Coutinho, Elsimar M. (1972). "Control of fertility by monthly injections of a mixture of norgestrel and a long-acting estrogen". Contraception. 5 (5): 395–399. doi:10.1016/0010-7824(72)90031-5. ISSN 0010-7824. PMID 4650657.
- ^ Garza-Flores J, Fatinikun T, Hernandez L, Ramos I, Cardenas M, Menjivar M (July 1991). "A pilot study on the assessment of a progesterone/estradiol sustained release as once-a-month-injectable contraceptive". Contraception. 44 (1): 45–59. doi:10.1016/0010-7824(91)90105-O. PMID 1893701.
- ^ Garza-Flores J, Hall PE, Perez-Palacios G (1991). "Long-acting hormonal contraceptives for women". J. Steroid Biochem. Mol. Biol. 40 (4–6): 697–704. doi:10.1016/0960-0760(91)90293-E. PMID 1958567. S2CID 26021562.
- ^ Joseph William Goldzieher; Kenneth Fotherby (1994). Pharmacology of the contraceptive steroids. Raven Press. p. 154. ISBN 978-0-7817-0097-9.
- ^ Zañartu J, Rice-Wray E, Goldzieher JW (October 1966). "Fertility control with long-acting injectable steroids. A preliminary report". Obstet Gynecol. 28 (4): 513–5. PMID 5925038.
- ^ Harry Beckman (1967). The Year Book of Drug Therapy. Year Book Publishers.
Further reading
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.








