| |
| |
| Names | |
|---|---|
| IUPAC name
(2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol
| |
| Other names
Cianidanol
Cyanidanol (+)-catechin D-Catechin Catechinic acid Catechuic acid Cianidol Dexcyanidanol (2R,3S)-Catechin 2,3-trans-Catechin (2R,3S)-Flavan-3,3′,4′,5,7-pentol | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.297 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H14O6 | |
| Molar mass | 290.271 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 175 to 177 °C (347 to 351 °F; 448 to 450 K) |
| UV-vis (λmax) | 276 nm |
Chiral rotation ([α]D)
|
+14.0° |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Mutagenic for mammalian somatic cells, mutagenic for bacteria and yeast |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
(+)-catechin : 10,000 mg/kg in rat (RTECS) 10,000 mg/kg in mouse 3,890 mg/kg in rat (other source) |
| Safety data sheet (SDS) | sciencelab AppliChem[permanent dead link] |
| Pharmacology | |
| Oral | |
| Pharmacokinetics: | |
| Urines | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Catechin /ˈkætɪkɪn/ is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
The name of the catechin chemical family derives from catechu, which is the tannic juice or boiled extract of Mimosa catechu (Acacia catechu L.f).[1]
Chemistry

Catechin possesses two benzene rings (called the A and B rings) and a dihydropyran heterocycle (the C ring) with a hydroxyl group on carbon 3. The A ring is similar to a resorcinol moiety while the B ring is similar to a catechol moiety. There are two chiral centers on the molecule on carbons 2 and 3. Therefore, it has four diastereoisomers. Two of the isomers are in trans configuration and are called catechin and the other two are in cis configuration and are called epicatechin.
The most common catechin isomer is (+)-catechin. The other stereoisomer is (−)-catechin or ent-catechin. The most common epicatechin isomer is (−)-epicatechin (also known under the names L-epicatechin, epicatechol, (−)-epicatechol, L-acacatechin, L-epicatechol, epicatechin, 2,3-cis-epicatechin or (2R,3R)-(−)-epicatechin).
The different epimers can be separated using chiral column chromatography.[2]
Making reference to no particular isomer, the molecule can just be called catechin. Mixtures of the different enantiomers can be called (±)-catechin or DL-catechin and (±)-epicatechin or DL-epicatechin.
Catechin and epicatechin are the building blocks of the proanthocyanidins, a type of condensed tannin.
- Diastereoisomers gallery
-
(+)-catechin (2R,3S)
-
(−)-catechin (2S,3R)
-
(−)-epicatechin (2R,3R)
-
(+)-epicatechin (2S,3S)

Moreover, the flexibility of the C-ring allows for two conformation isomers, putting the B-ring either in a pseudoequatorial position (E conformer) or in a pseudoaxial position (A conformer). Studies confirmed that (+)-catechin adopts a mixture of A- and E-conformers in aqueous solution and their conformational equilibrium has been evaluated to be 33:67.[3]
As flavonoids, catechins can act as antioxidants when in high concentration in vitro, but compared with other flavonoids, their antioxidant potential is low.[4] The ability to quench singlet oxygen seems to be in relation with the chemical structure of catechin, with the presence of the catechol moiety on ring B and the presence of a hydroxyl group activating the double bond on ring C.[5]
Oxidation
Electrochemical experiments show that (+)-catechin oxidation mechanism proceeds in sequential steps, related with the catechol and resorcinol groups and the oxidation is pH-dependent. The oxidation of the catechol 3′,4′-dihydroxyl electron-donating groups occurs first, at very low positive potentials, and is a reversible reaction. The hydroxyl groups of the resorcinol moiety oxidised afterwards were shown to undergo an irreversible oxidation reaction.[6]
The laccase/ABTS system oxidizes (+)-catechin to oligomeric products[7] of which proanthocyanidin A2 is a dimer.
Spectral data

| UV-Vis | |
|---|---|
| Lambda-max: | 276 nm |
| Extinction coefficient (log ε) | 4.01 |
| IR | |
| Major absorption bands | 1600 cm−1(benzene rings) |
| NMR | |
| Proton NMR
|
δ : 2.49 (1H, dd, J = 16.0, 8.6 Hz, H-4a), |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
ESI-MS [M+H]+ m/z : 291.0
|
Natural occurrences
(+)-Catechin and (−)-epicatechin as well as their gallic acid conjugates are ubiquitous constituents of vascular plants, and frequent components of traditional herbal remedies, such as Uncaria rhynchophylla. The two isomers are mostly found as cacao and tea constituents, as well as in Vitis vinifera grapes.[9][10][11]
In food
The main dietary sources of catechins in Europe and the United States are tea and pome fruits.[12][13]
Catechins and epicatechins are found in cocoa,[14] which, according to one database, has the highest content (108 mg/100 g) of catechins among foods analyzed, followed by prune juice (25 mg/100 ml) and broad bean pod (16 mg/100 g).[15] Açaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), contains (+)-catechins (67 mg/kg).[16]
Catechins are diverse among foods,[15] from peaches[17] to green tea and vinegar.[15][18] Catechins are found in barley grain, where they are the main phenolic compound responsible for dough discoloration.[19] The taste associated with monomeric (+)-catechin or (−)-epicatechin is described as slightly astringent, but not bitter.[20]
Metabolism
Biosynthesis
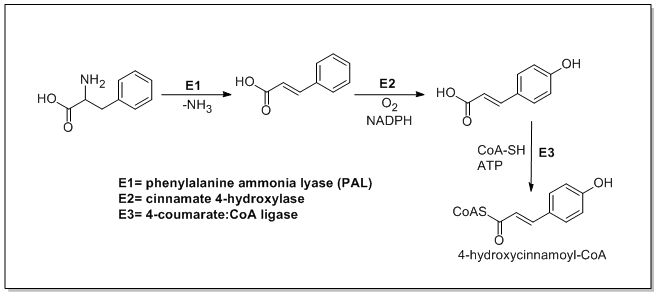
The biosynthesis of catechin begins with ma 4-hydroxycinnamoyl CoA starter unit which undergoes chain extension by the addition of three malonyl-CoAs through a PKSIII pathway. 4-Hydroxycinnamoyl CoA is biosynthesized from L-phenylalanine through the Shikimate pathway. L-Phenylalanine is first deaminated by phenylalanine ammonia lyase (PAL) forming cinnamic acid which is then oxidized to 4-hydroxycinnamic acid by cinnamate 4-hydroxylase. Chalcone synthase then catalyzes the condensation of 4-hydroxycinnamoyl CoA and three molecules of malonyl-CoA to form chalcone. Chalcone is then isomerized to naringenin by chalcone isomerase which is oxidized to eriodictyol by flavonoid 3′-hydroxylase and further oxidized to taxifolin by flavanone 3-hydroxylase. Taxifolin is then reduced by dihydroflavanol 4-reductase and leucoanthocyanidin reductase to yield catechin. The biosynthesis of catechin is shown below[21][22][23]
Leucocyanidin reductase (LCR) uses 2,3-trans-3,4-cis-leucocyanidin to produce (+)-catechin and is the first enzyme in the proanthocyanidin (PA) specific pathway. Its activity has been measured in leaves, flowers, and seeds of the legumes Medicago sativa, Lotus japonicus, Lotus uliginosus, Hedysarum sulfurescens, and Robinia pseudoacacia.[24] The enzyme is also present in Vitis vinifera (grape).[25]
Biodegradation
Catechin oxygenase, a key enzyme in the degradation of catechin, is present in fungi and bacteria.[26]
Among bacteria, degradation of (+)-catechin can be achieved by Acinetobacter calcoaceticus. Catechin is metabolized to protocatechuic acid (PCA) and phloroglucinol carboxylic acid (PGCA).[27] It is also degraded by Bradyrhizobium japonicum. Phloroglucinol carboxylic acid is further decarboxylated to phloroglucinol, which is dehydroxylated to resorcinol. Resorcinol is hydroxylated to hydroxyquinol. Protocatechuic acid and hydroxyquinol undergo intradiol cleavage through protocatechuate 3,4-dioxygenase and hydroxyquinol 1,2-dioxygenase to form β-carboxy-cis,cis-muconic acid and maleyl acetate.[28]
Among fungi, degradation of catechin can be achieved by Chaetomium cupreum.[29]
Metabolism in humans


Catechins are metabolised upon uptake from the gastrointestinal tract, in particular the jejunum,[31] and in the liver, resulting in so-called structurally related epicatechin metabolites (SREM).[32] The main metabolic pathways for SREMs are glucuronidation, sulfation and methylation of the catechol group by catechol-O-methyl transferase, with only small amounts detected in plasma.[33][30] The majority of dietary catechins are however metabolised by the colonic microbiome to gamma-valerolactones and hippuric acids which undergo further biotransformation, glucuronidation, sulfation and methylation in the liver.[33]
The stereochemical configuration of catechins has a strong impact on their uptake and metabolism as uptake is highest for (−)-epicatechin and lowest for (−)-catechin.[34]
Biotransformation
Biotransformation of (+)-catechin into taxifolin by a two-step oxidation can be achieved by Burkholderia sp.[35]
(+)-Catechin and (−)-epicatechin are transformed by the endophytic filamentous fungus Diaporthe sp. into the 3,4-cis-dihydroxyflavan derivatives, (+)-(2R,3S,4S)-3,4,5,7,3′,4′-hexahydroxyflavan (leucocyanidin) and (−)-(2R,3R,4R)-3,4,5,7,3′,4′-hexahydroxyflavan, respectively, whereas (−)-catechin and (+)-epicatechin with a (2S)-phenyl group resisted the biooxidation.[36]
Leucoanthocyanidin reductase (LAR) uses (2R,3S)-catechin, NADP+ and H2O to produce 2,3-trans-3,4-cis-leucocyanidin, NADPH, and H+. Its gene expression has been studied in developing grape berries and grapevine leaves.[37]
Glycosides
- (2R,3S)-Catechin-7-O-β-D-glucopyranoside can be isolated from barley (Hordeum vulgare L.) and malt.[38]
- Epigeoside (catechin-3-O-α-L-rhamnopyranosyl-(1–4)-β-D-glucopyranosyl-(1–6)-β-D-glucopyranoside) can be isolated from the rhizomes of Epigynum auritum.[39]
Research

Vascular function
Only limited evidence from dietary studies indicates that catechins may affect endothelium-dependent vasodilation which could contribute to normal blood flow regulation in humans.[40][41] Green tea catechins may improve blood pressure, especially when systolic blood pressure is above 130 mmHg.[42][43]
Due to extensive metabolism during digestion, the fate and activity of catechin metabolites responsible for this effect on blood vessels, as well as the actual mode of action, are unknown.[33][44]
Adverse events
Catechin and its metabolites can bind tightly to red blood cells and thereby induce the development of autoantibodies, resulting in haemolytic anaemia and renal failure.[45] This resulted in the withdrawal of the catechin-containing drug Catergen, used to treat viral hepatitis,[46] from market in 1985.[47]
Catechins from green tea can be hepatotoxic[48] and the European Food Safety Authority has recommended not to exceed 800 mg per day.[49]
Other
One limited meta-analysis showed that increasing consumption of green tea and its catechins to seven cups per day provided a small reduction in prostate cancer.[50] Nanoparticle methods are under preliminary research as potential delivery systems of catechins.[51]
Botanical effects
Catechins released into the ground by some plants may hinder the growth of their neighbors, a form of allelopathy.[52] Centaurea maculosa, the spotted knapweed often studied for this behavior, releases catechin isomers into the ground through its roots, potentially having effects as an antibiotic or herbicide. One hypothesis is that it causes a reactive oxygen species wave through the target plant's root to kill root cells by apoptosis.[53] Most plants in the European ecosystem have defenses against catechin, but few plants are protected against it in the North American ecosystem where Centaurea maculosa is an invasive, uncontrolled weed.[52]
Catechin acts as an infection-inhibiting factor in strawberry leaves.[54] Epicatechin and catechin may prevent coffee berry disease by inhibiting appressorial melanization of Colletotrichum kahawae.[55]
References
- ^ "Cutch and catechu plant origin". Food and Agriculture Organization of the United Nations. 5 November 2011. Archived from the original on 10 February 2019. Retrieved 26 July 2016.
- ^ Rinaldo D, Batista JM, Rodrigues J, Benfatti AC, Rodrigues CM, dos Santos LC, et al. (August 2010). "Determination of catechin diastereomers from the leaves of Byrsonima species using chiral HPLC-PAD-CD". Chirality. 22 (8): 726–733. doi:10.1002/chir.20824. PMID 20143413.
- ^ Kríz Z, Koca J, Imberty A, Charlot A, Auzély-Velty R (July 2003). "Investigation of the complexation of (+)-catechin by beta-cyclodextrin by a combination of NMR, microcalorimetry and molecular modeling techniques". Organic & Biomolecular Chemistry. 1 (14): 2590–2595. doi:10.1039/B302935M. PMID 12956082.
- ^ Pietta PG (July 2000). "Flavonoids as antioxidants". Journal of Natural Products. 63 (7): 1035–1042. doi:10.1021/np9904509. PMID 10924197. S2CID 23310671.
- ^ Tournaire C, Croux S, Maurette MT, Beck I, Hocquaux M, Braun AM, Oliveros E (August 1993). "Antioxidant activity of flavonoids: efficiency of singlet oxygen (1Δg) quenching". Journal of Photochemistry and Photobiology. B, Biology. 19 (3): 205–215. doi:10.1016/1011-1344(93)87086-3. PMID 8229463.
- ^ Janeiro P, Oliveira Brett AM (2004). "Catechin electrochemical oxidation mechanisms". Analytica Chimica Acta. 518 (1–2): 109–115. Bibcode:2004AcAC..518..109J. doi:10.1016/j.aca.2004.05.038. hdl:10316/5128.
- ^ Osman AM, Wong KK, Fernyhough A (April 2007). "The laccase/ABTS system oxidizes (+)-catechin to oligomeric products". Enzyme and Microbial Technology. 40 (5): 1272–1279. doi:10.1016/j.enzmictec.2006.09.018.
- ^ Lin YP, Chen TY, Tseng HW, Lee MH, Chen ST (June 2009). "Neural cell protective compounds isolated from Phoenix hanceana var. formosana". Phytochemistry. 70 (9): 1173–1181. Bibcode:2009PChem..70.1173L. doi:10.1016/j.phytochem.2009.06.006. PMID 19628235. S2CID 28636157.
- ^ Aizpurua-Olaizola O, Ormazabal M, Vallejo A, Olivares M, Navarro P, Etxebarria N, Usobiaga A (January 2015). "Optimization of supercritical fluid consecutive extractions of fatty acids and polyphenols from Vitis vinifera grape wastes". Journal of Food Science. 80 (1): E101–E107. doi:10.1111/1750-3841.12715. PMID 25471637.
- ^ Freudenberg K, Cox RF, Braun E (1932). "The Catechin of the Cacao Bean1". Journal of the American Chemical Society. 54 (5): 1913–1917. doi:10.1021/ja01344a026.
- ^ "Michiyo Tsujimura (1888–1969)". Archived from the original on 21 November 2015. Retrieved 10 November 2015.
- ^ Chun OK, Chung SJ, Song WO (May 2007). "Estimated dietary flavonoid intake and major food sources of U.S. adults". The Journal of Nutrition. 137 (5): 1244–1252. doi:10.1093/jn/137.5.1244. PMID 17449588.
- ^ Vogiatzoglou A, Mulligan AA, Lentjes MA, Luben RN, Spencer JP, Schroeter H, et al. (2015). "Flavonoid intake in European adults (18 to 64 years)". PLOS ONE. 10 (5): e0128132. Bibcode:2015PLoSO..1028132V. doi:10.1371/journal.pone.0128132. PMC 4444122. PMID 26010916.
- ^ Kwik-Uribe C, Bektash RM (2008). "Cocoa flavanols - measurement, bioavailability and bioactivity" (PDF). Asia Pacific Journal of Clinical Nutrition. 17 (Suppl. 1): 280–283. PMID 18296356.
- ^ a b c "Polyphenols in green tea infusion". Phenol-Explorer, v 3.5. 2014. Retrieved 1 November 2014.
- ^ Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (June 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açaí (Euterpe oleracea Mart.)". Journal of Agricultural and Food Chemistry. 56 (12): 4631–4636. doi:10.1021/jf800161u. PMID 18522407.
- ^ Cheng GW, Crisosto CH (1995). "Browning Potential, Phenolic Composition, and Polyphenoloxidase Activity of Buffer Extracts of Peach and Nectarine Skin Tissue". Journal of the American Society for Horticultural Science. 120 (5): 835–838. doi:10.21273/JASHS.120.5.835.
- ^ Gálvez MC, Barroso CG, Pérez-Bustamante JA (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199 (1): 29–31. doi:10.1007/BF01192948. S2CID 91784893.
- ^ Quinde-Axtell Z, Baik BK (December 2006). "Phenolic compounds of barley grain and their implication in food product discoloration". Journal of Agricultural and Food Chemistry. 54 (26): 9978–9984. doi:10.1021/jf060974w. PMID 17177530.
- ^ Kielhorn, S.; Thorngate, J. H. III (1999). "Oral sensations associated with the flavan-3-ols (+)-catechin and (−)-epicatechin". Food Quality and Preference. 10 (2): 109–116. doi:10.1016/S0950-3293(98)00049-4.
- ^ Rani A, Singh K, Ahuja PS, Kumar S (March 2012). "Molecular regulation of catechins biosynthesis in tea [Camellia sinensis (L.) O. Kuntze]". Gene. 495 (2): 205–210. doi:10.1016/j.gene.2011.12.029. PMID 22226811.
- ^ Punyasiri PA, Abeysinghe IS, Kumar V, Treutter D, Duy D, Gosch C, et al. (November 2004). "Flavonoid biosynthesis in the tea plant Camellia sinensis: properties of enzymes of the prominent epicatechin and catechin pathways". Archives of Biochemistry and Biophysics. 431 (1): 22–30. doi:10.1016/j.abb.2004.08.003. PMID 15464723.
- ^ Dewick PM (2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). UK: John Wiley & Sons. ISBN 978-0-470-74167-2.[page needed]
- ^ Skadhauge B, Gruber MY, Thomsen KK, Von Wettstein D (April 1997). "Leucocyanidin Reductase Activity and Accumulation of Proanthocyanidins in Developing Legume Tissues". American Journal of Botany. 84 (4): 494–503. doi:10.2307/2446026. JSTOR 2446026.
- ^ Maugé C, Granier T, d'Estaintot BL, Gargouri M, Manigand C, Schmitter JM, et al. (April 2010). "Crystal structure and catalytic mechanism of leucoanthocyanidin reductase from Vitis vinifera". Journal of Molecular Biology. 397 (4): 1079–1091. doi:10.1016/j.jmb.2010.02.002. PMID 20138891.
- ^ Arunachalam, M.; Mohan Raj, M.; Mohan, N.; Mahadevan, A. (2003). "Biodegradation of Catechin" (PDF). Proceedings of the Indian National Science Academy. B69 (4): 353–370. Archived from the original (PDF) on 2012-03-16.
- ^ Arunachalam M, Mohan N, Sugadev R, Chellappan P, Mahadevan A (June 2003). "Degradation of (+)-catechin by Acinetobacter calcoaceticus MTC 127". Biochimica et Biophysica Acta (BBA) - General Subjects. 1621 (3): 261–265. doi:10.1016/S0304-4165(03)00077-1. PMID 12787923.
- ^ Hopper W, Mahadevan A (1997). "Degradation of catechin by Bradyrhizobium japonicum". Biodegradation. 8 (3): 159–165. doi:10.1023/A:1008254812074. S2CID 41221044.
- ^ Sambandam T, Mahadevan A (January 1993). "Degradation of catechin and purification and partial characterization of catechin oxygenase from Chaetomium cupreum". World Journal of Microbiology & Biotechnology. 9 (1): 37–44. doi:10.1007/BF00656513. PMID 24419836. S2CID 1257624.
- ^ a b c d Ottaviani JI, Borges G, Momma TY, Spencer JP, Keen CL, Crozier A, Schroeter H (July 2016). "The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives". Scientific Reports. 6: 29034. Bibcode:2016NatSR...629034O. doi:10.1038/srep29034. PMC 4929566. PMID 27363516.
- ^ Actis-Goretta L, Lévèques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. (October 2013). "Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique". The American Journal of Clinical Nutrition. 98 (4): 924–933. doi:10.3945/ajcn.113.065789. PMID 23864538.
- ^ Ottaviani JI, Momma TY, Kuhnle GK, Keen CL, Schroeter H (April 2012). "Structurally related (−)-epicatechin metabolites in humans: assessment using de novo chemically synthesized authentic standards". Free Radical Biology & Medicine. 52 (8): 1403–1412. doi:10.1016/j.freeradbiomed.2011.12.010. PMID 22240152.
- ^ a b c "Flavonoids". Linus Pauling Institute, Oregon State University, Corvallis. 2016. Retrieved 24 July 2016.
- ^ Ottaviani JI, Momma TY, Heiss C, Kwik-Uribe C, Schroeter H, Keen CL (January 2011). "The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo". Free Radical Biology & Medicine. 50 (2): 237–244. doi:10.1016/j.freeradbiomed.2010.11.005. PMID 21074608.
- ^ Matsuda M, Otsuka Y, Jin S, Wasaki J, Watanabe J, Watanabe T, Osaki M (February 2008). "Biotransformation of (+)-catechin into taxifolin by a two-step oxidation: primary stage of (+)-catechin metabolism by a novel (+)-catechin-degrading bacteria, Burkholderia sp. KTC-1, isolated from tropical peat". Biochemical and Biophysical Research Communications. 366 (2): 414–419. doi:10.1016/j.bbrc.2007.11.157. PMID 18068670.
- ^ Shibuya H, Agusta A, Ohashi K, Maehara S, Simanjuntak P (July 2005). "Biooxidation of (+)-catechin and (−)-epicatechin into 3,4-dihydroxyflavan derivatives by the endophytic fungus Diaporthe sp. isolated from a tea plant". Chemical & Pharmaceutical Bulletin. 53 (7): 866–867. doi:10.1248/cpb.53.866. PMID 15997157.
- ^ Bogs J, Downey MO, Harvey JS, Ashton AR, Tanner GJ, Robinson SP (October 2005). "Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves". Plant Physiology. 139 (2): 652–663. doi:10.1104/pp.105.064238. JSTOR 4281902. PMC 1255985. PMID 16169968.
- ^ Friedrich W, Galensa R (2002). "Identification of a new flavanol glucoside from barley (Hordeum vulgare L.) and malt". European Food Research and Technology. 214 (5): 388–393. doi:10.1007/s00217-002-0498-x. S2CID 84221785.
- ^ Jin QD, Mu QZ (1991). "[Study on glycosidal constituents from Epigynum auritum]". Yao Xue Xue Bao (Acta Pharmaceutica Sinica) (in Chinese). 26 (11): 841–845. PMID 1823978.
- ^ Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A (March 2012). "Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials". The American Journal of Clinical Nutrition. 95 (3): 740–751. doi:10.3945/ajcn.111.023457. PMID 22301923.
- ^ Ellinger S, Reusch A, Stehle P, Helfrich HP (June 2012). "Epicatechin ingested via cocoa products reduces blood pressure in humans: a nonlinear regression model with a Bayesian approach". The American Journal of Clinical Nutrition. 95 (6): 1365–1377. doi:10.3945/ajcn.111.029330. PMID 22552030.
- ^ Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht-Nasrabadi E, Khosravi-Boroujeni H (September 2014). "Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials". European Journal of Nutrition. 53 (6): 1299–1311. doi:10.1007/s00394-014-0720-1. PMID 24861099. S2CID 206969226.
- ^ Aprotosoaie AC, Miron A, Trifan A, Luca VS, Costache II (December 2016). "The Cardiovascular Effects of Cocoa Polyphenols-An Overview". Diseases. 4 (4): 39. doi:10.3390/diseases4040039. PMC 5456324. PMID 28933419.
- ^ Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, et al. (January 2006). "(−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans". Proceedings of the National Academy of Sciences of the United States of America. 103 (4): 1024–1029. Bibcode:2006PNAS..103.1024S. doi:10.1073/pnas.0510168103. PMC 1327732. PMID 16418281.
- ^ Martinez SE, Davies NM, Reynolds JK (2013). "Toxicology and Safety of Flavonoids". Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety, and Toxicology. John Wiley & Son. p. 257. ISBN 978-0-470-57871-1.
- ^ Bode JC (1987). Okolicsányi L, Csomós G, Crepaldi G (eds.). Assessment and Management of Hepatobiliary Disease. Berlin: Springer-Verlag. p. 371. doi:10.1007/978-3-642-72631-6. ISBN 978-3-642-72631-6. S2CID 3167832.
- ^ "Ruhen der Zulassung für Catergen" (PDF). Deutsches Ärzteblatt. 82 (38): 2706.
- ^ Health Canada (2017-11-15). "Summary Safety Review - Green tea extract-containing natural health products - Assessing the potential risk of liver injury (hepatotoxicity)". www.canada.ca. Retrieved 2022-05-06.
- ^ Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, et al. (April 2018). "Scientific opinion on the safety of green tea catechins". EFSA Journal. 16 (4): e05239. doi:10.2903/j.efsa.2018.5239. PMC 7009618. PMID 32625874.
- ^ Guo Y, Zhi F, Chen P, Zhao K, Xiang H, Mao Q, et al. (March 2017). "Green tea and the risk of prostate cancer: A systematic review and meta-analysis". Medicine. 96 (13): e6426. doi:10.1097/MD.0000000000006426. PMC 5380255. PMID 28353571.
- ^ Ye JH, Augustin MA (2018). "Nano- and micro-particles for delivery of catechins: Physical and biological performance". Critical Reviews in Food Science and Nutrition. 59 (10): 1563–1579. doi:10.1080/10408398.2017.1422110. PMID 29345975. S2CID 29522787.
- ^ a b Broz AK, Vivanco JM, Schultz MJ, Perry LG, Paschke MW (2006). "Secondary Metabolites and Allelopathy in Plant Invasions: A Case Study of Centaurea maculosa". In Taiz L, Zeiger E, Møller IM, Murphy A (eds.). Plant Physiology and Development (6th ed.). Sinauer Associates.
- ^ Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (September 2003). "Allelopathy and exotic plant invasion: from molecules and genes to species interactions". Science. 301 (5638): 1377–1380. Bibcode:2003Sci...301.1377B. doi:10.1126/science.1083245. PMID 12958360. S2CID 26483595.
- ^ Yamamoto M, Nakatsuka S, Otani H, Kohmoto K, Nishimura S (June 2000). "(+)-Catechin acts as an infection-inhibiting factor in strawberry leaf". Phytopathology. 90 (6): 595–600. doi:10.1094/PHYTO.2000.90.6.595. PMID 18944538.
- ^ Chen Z, Liang J, Zhang C, Rodrigues CJ (October 2006). "Epicatechin and catechin may prevent coffee berry disease by inhibition of appressorial melanization of Colletotrichum kahawae". Biotechnology Letters. 28 (20): 1637–1640. doi:10.1007/s10529-006-9135-2. PMID 16955359. S2CID 30593181.
External links
 Media related to (+)-Catechin at Wikimedia Commons
Media related to (+)-Catechin at Wikimedia Commons