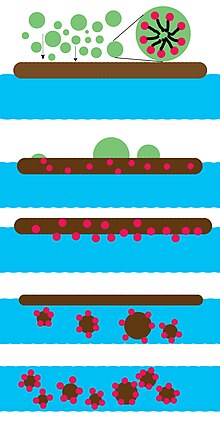
An oil dispersant is a mixture of emulsifiers and solvents that helps break oil into small droplets following an oil spill. Small droplets are easier to disperse throughout a water volume, and small droplets may be more readily biodegraded by microbes in the water. Dispersant use involves a trade-off between exposing coastal life to surface oil and exposing aquatic life to dispersed oil. While submerging the oil with dispersant may lessen exposure to marine life on the surface, it increases exposure for animals dwelling underwater, who may be harmed by toxicity of both dispersed oil and dispersant.[1][2][3] Although dispersant reduces the amount of oil that lands ashore, it may allow faster, deeper penetration of oil into coastal terrain, where it is not easily biodegraded.[4]
History

Torrey Canyon
In 1967, the supertanker Torrey Canyon leaked oil onto the English coastline.[5] Alkylphenol surfactants were primarily used to break up the oil, but proved very toxic in the marine environment; all types of marine life were killed. This led to a reformulation of dispersants to be more environmentally sensitive.[when?] After the Torrey Canyon spill, new boat-spraying systems were developed.[5] Later reformulations allowed more dispersant to be contained (at a higher concentration) to be aerosolized.
Exxon Valdez
Alaska had fewer than 4,000 gallons of dispersants available at the time of the Exxon Valdez oil spill, and no aircraft with which to dispense them. The dispersants introduced were relatively ineffective due to insufficient wave action to mix the oil and water, and their use was shortly abandoned.[6]
A report by David Kirby for TakePart found that the main component of the Corexit 9527 formulation used during Exxon Valdez cleanup, 2-butoxyethanol, was identified as "one of the agents that caused liver, kidney, lung, nervous system, and blood disorders among cleanup crews in Alaska following the 1989 Exxon Valdez spill."[7]
Early use (by volume)
Dispersants were applied to a number of oil spills between the years 1967 and 1989.[8]
| Year | Spill | Country | Oil volume (L) | Dispersant volume (L) |
|---|---|---|---|---|
| 1967 | Torrey Canyon | England | 119,000,000 | 10,000,000 |
| 1968 | Ocean Eagle | Puerto Rico | 12,000,000 | 6,000 |
| 1969 | Santa Barbara | USA | 1,000,000 | 3,200 |
| 1970 | Arrow | Canada | 5,000,000 | 1,200 |
| 1970 | Pacific Glory | England | 6,300,000 | |
| 1975 | Showa Maru | Singapore | 15,000,000 | 500,000 |
| 1975 | Jakob Maersk | Portugal | 88,000,000 | 110,000 |
| 1976 | Urquiola | Spain | 100,000,000 | 2,400,000 |
| 1978 | Amoco Cadiz | France | 200,000,000 | 2,500,000 |
| 1978 | Eleni V | England | 7,500,000 | 900,000 |
| 1978 | Christos Bitas | England | 3,000,000 | 280,000 |
| 1979 | Betelgeuse | Ireland | 10,000,000 | 35,000 |
| 1979 | Ixtoc I | Mexico | 500,000,000 | 5,000,000 |
| 1983 | Sivand | England | 6,000,000 | 110,000 |
| 1984 | SS Puerto Rican | USA | 7,570[9] | |
| 1989 | Exxon Valdez | USA | 50,000,000 | 8,000 |
Deepwater Horizon
During the Deep water Horizon oil spill, an estimated 1.84 million gallons of Corexit was used in an attempt to increase the amount of surface oil and mitigate the damage to coastal habitat. BP purchased all of the world's supply of Corexit soon after the spill began.[10] Nearly half (771,000 gallons) of the dispersants were applied directly at the wellhead.[11] The primary dispersant used were Corexit 9527 and 9500, which were controversial due to toxicity.
In 2012, a study found that Corexit made the oil up to 52 times more toxic than oil alone,[12] and that the dispersant's emulsifying effect makes oil droplets more bio-available to plankton.[13] The Georgia Institute of Technology found that "Mixing oil with dispersant increased toxicity to ecosystems" and made the gulf oil spill worse.[14]
In 2013, in response to the growing body of laboratory-derived toxicity data, some researchers address the scrutiny that should be used when evaluating laboratory test results that have been extrapolated using procedures that are not fully reliable for environmental assessments.[15][16] Since then, guidance has been published that improves the comparability and relevance of oil toxicity tests.[17]
Rena oil spill
Maritime New Zealand used the oil dispersant Corexit 9500 to help in the cleanup process.[18] The dispersant was applied for only a week, after results proved inconclusive.[19]
Theory
Overview
Surfactants reduce oil-water interfacial tension, which helps waves break oil into small droplets. A mixture of oil and water is normally unstable, but can be stabilized with the addition of surfactants; these surfactants can prevent coalescence of dispersed oil droplets. The effectiveness of the dispersant depends on the weathering of the oil, sea energy (waves), salinity of the water, temperature and the type of oil.[20] Dispersion is unlikely to occur if the oil spreads into a thin layer, because the dispersant requires a particular thickness to work; otherwise, the dispersant will interact with both the water and the oil. More dispersant may be required if the sea energy is low. The salinity of the water is more important for ionic-surfactant dispersants, as salt screens electrostatic interactions between molecules. The viscosity of the oil is another important factor; viscosity can retard dispersant migration to the oil-water interface and also increase the energy required to shear a drop from the slick. Viscosities below 2,000 centipoise are optimal for dispersants. If the viscosity is above 10,000 centipoise, no dispersion is possible.[21]
Requirements
There are five requirements for surfactants to successfully disperse oil:[5]
- Dispersant must be on the oil's surface in the proper concentration
- Dispersant must penetrate (mix with) the oil
- Surfactant molecules must orient at the oil-water interface (hydrophobic in oil and hydrophilic in water)
- Oil-water interfacial tension must be lowered (so the oil can be broken up).
- Energy must be applied to the mix (for example, by waves)
Effectiveness
The effectiveness of a dispersant may be analyzed with the following equations.[22] The Area refers to the area under the absorbance/wavelength curve, which is determined using the trapezoidal rule. The absorbances are measured at 340, 370, and 400 nm.
Area = 30(Abs340 + Abs370)/2 + 30(Abs340 + Abs400)/2 (1)
The dispersant effectiveness may then be calculated using the equation below.
Effectiveness (%) = Total oil dispersed x 100/(ρoilVoil)
- ρoil = density of the test oil (g/L)
- Voil = volume of oil added to test flask (L)
- Total oil dispersed = mass of oil x 120mL/30mL
- Mass of oil = concentration oil x VDCM
- VDCM = final volume of DCM-extract of water sample (0.020 L)
- Concentration of oil = area determined by Equation (1) / slope of calibration curve
Dispersion models
Developing well-constructed models (accounting for variables such as oil type, salinity and surfactant) are necessary to select the appropriate dispersant in a given situation. Two models exist which integrate the use of dispersants: Mackay's model and Johansen's model.[23] There are several parameters which must be considered when creating a dispersion model, including oil-slick thickness, advection, resurfacing and wave action.[23] A general problem in modeling dispersants is that they change several of these parameters; surfactants lower the thickness of the film, increase the amount of diffusion into the water column and increase the amount of breakup caused by wave action. This causes the oil slick's behavior to be more dominated by vertical diffusion than horizontal advection.[23]
One equation for the modeling of oil spills is:[24]
where
- h is the oil-slick thickness
- is the velocity of ocean currents in the mixing layer of the water column (where oil and water mix together)
- is the wind-driven shear stress
- f is the oil-water friction coefficient
- E is the relative difference in densities between the oil and water
- R is the rate of spill propagation
Mackay's model predicts an increasing dispersion rate, as the slick becomes thinner in one dimension. The model predicts that thin slicks will disperse faster than thick slicks for several reasons. Thin slicks are less effective at dampening waves and other sources of turbidity. Additionally, droplets formed upon dispersion are expected to be smaller in a thin slick and thus easier to disperse in water. The model also includes:[23]
- An expression for the diameter of the oil drop
- Temperature dependence of oil movement
- An expression for the resurfacing of oil
- Calibrations based on data from experimental spills
The model is lacking in several areas: it does not account for evaporation, the topography of the ocean floor or the geography of the spill zone.[23]
Johansen's model is more complex than Mackay's model. It considers particles to be in one of three states: at the surface, entrained in the water column or evaporated. The empirically based model uses probabilistic variables to determine where the dispersant will move and where it will go after it breaks up oil slicks. The drift of each particle is determined by the state of that particle; this means that a particle in the vapor state will travel much further than a particle on the surface (or under the surface) of the ocean.[23] This model improves on Mackay's model in several key areas, including terms for:[23]
- Probability of entrainment – depends on wind
- Probability of resurfacing – depends on density, droplet size, time submerged and wind
- Probability of evaporation – matched with empirical data
Oil dispersants are modeled by Johansen using a different set of entrainment and resurfacing parameters for treated versus untreated oil. This allows areas of the oil slick to be modeled differently, to better understand how oil spreads along the water's surface.
Surfactants
Surfactants are classified into four main types, each with different properties and applications: anionic, cationic, nonionic and zwitterionic (or amphoteric). Anionic surfactants are compounds that contain an anionic polar group. Examples of anionic surfactants include sodium dodecyl sulfate and dioctyl sodium sulfosuccinate.[25] Included in this class of surfactants are sodium alkylcarboxylates (soaps).[26] Cationic surfactats are similar in nature to anionic surfactants, except the surfactant molecules carry a positive charge at the hydrophilic portion. Many of these compounds are quaternary ammonium salts, as well as cetrimonium bromide (CTAB).[26] Non-ionic surfactants are non-charged and together with anionic surfactants make up the majority of oil-dispersant formulations.[25] The hydrophilic portion of the surfactant contains polar functional groups, such as -OH or -NH.[26] Zwitterionic surfactants are the most expensive, and are used for specific applications.[26] These compounds have both positively and negatively charged components. An example of a zwitterionic compound is phosphatidylcholine, which as a lipid is largely insoluble in water.[26]
HLB values
Surfactant behavior is highly dependent on the hydrophilic-lipophilic balance (HLB) value. The HLB is a coding scale from 0 to 20 for non-ionic surfactants, and takes into account the chemical structure of the surfactant molecule. A zero value corresponds to the most lipophilic and a value of 20 is the most hydrophilic for a non-ionic surfactant.[5] In general, compounds with an HLB between one and four will not mix with water. Compounds with an HLB value above 13 will form a clear solution in water.[25] Oil dispersants usually have HLB values from 8–18.[25]
| Surfactant | Structure | Avg mol wt | HLB |
|---|---|---|---|
| Arkopal N-300 | C9H19C6H4O(CH2CH2O)30H | 1,550 | 17.0[27] |
| Brij 30 | polyoxyethylenated straight chain alcohol | 362 | 9.7[28] |
| Brij 35 | C12H25O(CH2CH2O)23H | 1,200 | 17.0[27] |
| Brij 56 | C16H33O(CH2CH2O)10H | 682 | 12.9[29] |
| Brij 58 | C16H33O(CH2CH2O)20H | 1122 | 15.7[29] |
| EGE Coco | ethyl glucoside | 415 | 10.6[28] |
| EGE no. 10 | ethyl glucoside | 362 | 12.5[28] |
| Genapol X-150 | C13H27O(CH2CH2O)15H | 860 | 15.0[27] |
| Tergitol NP-10 | nonylphenolethoxylate | 682 | 13.6[28] |
| Marlipal 013/90 | C13H27O(CH2CH2O)9H | 596 | 13.3[27] |
| Pluronic PE6400 | HO(CH2CH2O)x(C2H4CH2O)30(CH2CH2O)28-xH | 3000 | N.A.[27] |
| Sapogenat T-300 | (C4H9)3C6H2O(CH2CH2O)30H | 1600 | 17.0[27] |
| T-Maz 60K | ethoxylated sorbitan monostearate | 1310 | 14.9[28] |
| T-Maz 20 | ethoxylated sorbitan monolaurate | 1226 | 16.7[28] |
| Triton X-45 | C8H17C6H4O(CH2CH2O)5H | 427 | 10.4[29] |
| Triton X-100 | C8H17C6H4(OC2H4)10OH | 625 | 13.6[30] |
| Triton X-102 | C8H17C6H4O(CH2CH2O)12H | 756 | 14.6[27] |
| Triton X-114 | C8H17C6H4O(CH2CH2O)7.5H | 537 | 12.4[29] |
| Triton X-165 | C8H17C6H4O(CH2CH2O)16H | 911 | 15.8[29] |
| Tween 80 | C18H37-C6H9O5-(OC2H4)20OH | 1309 | 13.4[30] |
Comparative industrial formulations
Two formulations of different dispersing agents for oil spills, Dispersit and Omni-Clean, are shown below. A key difference between the two is that Omni-Clean uses ionic surfactants and Dispersit uses entirely non-ionic surfactants. Omni-Clean was formulated for little or no toxicity toward the environment. Dispersit, however, was designed as a competitor with Corexit. Dispersit contains non-ionic surfactants, which permit both primarily oil-soluble and primarily water-soluble surfactants. The partitioning of surfactants between the phases allows for effective dispersion.
| Omni-Clean OSD [31] | Dispersit [32] | ||||||
|---|---|---|---|---|---|---|---|
| Category | Ingredient | Function | Category | Ingredient | Function | ||
| Surfactant | Sodium lauryl sulfate | Charged ionic surfactant and thickener | Emulsifying agent | Oleic acid sorbitan monoester | Emulsifying agent | ||
| Surfactant | Cocamidopropyl betaine | Emulsifying agent | Surfactant | Coconut oil monoethanolamide | Dissolves oil and water into each other | ||
| Surfactant |  |
Ethoxylated nonylphenol | Petroleum emulsifier & wetting agent | Surfactant | Poly(ethylene glycol) monooleate | Oil-soluble surfactant | |
| Dispersant |  |
Lauric acid diethanolamide | Non-ionic viscosity booster & emulsifier | Surfactant |  |
Polyethoxylated tallow amine | Oil-soluble surfactant |
| Detergent | Diethanolamine | Water-soluble detergent for cutting oil | Surfactant |  |
Polyethoxylated linear secondary alcohol | Oil-soluble surfactant | |
| Emulsifier |  |
Propylene glycol | Solvent for oils, wetting agent, emulsifier | Solvent |  |
Dipropylene glycol methyl ether | Enhances solubility of surfactants in water and oil. |
| Solvent | H2O | Water | Reduces viscosity | Solvent | H2O | Water | Reduces viscosity |
Degradation and toxicity
Concerns regarding the persistence in the environment and toxicity to various flora and fauna of oil dispersants date back to their early use in the 1960s and 1970s.[33] Both the degradation and the toxicity of dispersants depend on the chemicals chosen within the formulation. Compounds which interact too harshly with oil dispersants should be tested to ensure that they meet three criteria:[34]
- They should be biodegradable.
- In the presence of oil, they must not be preferentially utilized as a carbon source.
- They must be nontoxic to indigenous bacteria.
Methods of use

Dispersants can be delivered in aerosolized form by an aircraft or boat. Sufficient dispersant with droplets in the proper size are necessary; this can be achieved with an appropriate pumping rate. Droplets larger than 1,000 μm are preferred, to ensure they are not blown away by the wind. The ratio of dispersant to oil is typically 1:20.[20]
See also
References
- ^ "Treating oil spills with chemical dispersants: Is the cure worse than the ailment?". Retrieved 7 April 2014.
- ^ "Dispersants EPA.gov".
- ^ "Dispersants". Center for Biological Diversity. Retrieved 6 April 2014.
- ^ "Study: Dispersants Can Move Hydrocarbons Faster and Deeper into Gulf Sand". 2013-05-10.
- ^ a b c d Clayton, John R. (1992). Oil Spill Dispersants: Mechanisms of Action and Laboratory Tests. C K Smoley & Sons. pp. 9–23. ISBN 978-0-87371-946-9.
- ^ EPA: Learning Center: Exxon Valdez. http://www.epa.gov/oem/content/learning/exxon.htm accessed 5/23/2012
- ^ "Corexit: An Oil Spill Solution Worse Than the Problem?". TakePart. Archived from the original on 5 May 2016. Retrieved 4 April 2014.
- ^ Jafvert, Chad (2011-09-23). "Understanding oil dispersants" (PDF). School of Civil Engineering & Division of Environmental and Ecological Engineering. Purdue University. Retrieved 2015-03-07.
- ^ "Tank from ship towed into port". Santa Cruz Sentinel. 1984-11-06. Retrieved 2015-03-08.
- ^ "Less Toxic Dispersants Lose Out in BP Oil Spill Cleanup". The New York Times. Retrieved 4 April 2014.
- ^ National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling. "Use of Surface and Subsea Dispersants During the BP Deepwater Horizon Oil Spill". Retrieved 23 May 2012.
- ^ GT | Newsroom - Gulf of Mexico Clean-Up Makes 2010 Spill 52-Times More Toxic
- ^ "Dispersant Makes Oil 52 Times More Toxic". Live Science. 30 November 2012.
- ^ Study: Mixing oil with dispersant made the BP oil spill worse | Science Recorder
- ^ Coelho, Gina; Clark, James; Aurand, Don (2013-06-01). "Toxicity testing of dispersed oil requires adherence to standardized protocols to assess potential real world effects". Environmental Pollution. 177: 185–188. doi:10.1016/j.envpol.2013.02.004. ISSN 1873-6424. PMID 23497795.
- ^ Bejarano, Adriana C.; Clark, James R.; Coelho, Gina M. (2014-04-01). "Issues and challenges with oil toxicity data and implications for their use in decision making: A quantitative review". Environmental Toxicology and Chemistry. 33 (4): 732–742. doi:10.1002/etc.2501. ISSN 1552-8618. PMID 24616123.
- ^ Redman, Aaron D.; Parkerton, Thomas F. (2015-09-15). "Guidance for improving comparability and relevance of oil toxicity tests". Marine Pollution Bulletin. 98 (1–2): 156–170. Bibcode:2015MarPB..98..156R. doi:10.1016/j.marpolbul.2015.06.053. PMID 26162510.
- ^ "Dispersants 'worse than oil'". 11 October 2011.
- ^ "Cleaning up the oil spill".
- ^ a b Fingas, Merv (2001). The Basics of Oil Spill Cleanup. Lewis Publishers. pp. 120–125. ISBN 978-1-56670-537-0.
- ^ National Research Council (U.S.) (1989). Using Oil Spill Dispersants on the Sea. Washington, D.C.: National Academy Press. p. 54.
- ^ Chandrasekar, Subhashini; Sorial, George; Weaver, James (2006). "Dispersant effectiveness on oil spills – impact of salinity". ICES Journal of Marine Science. 63 (8): 1418–1430. Bibcode:2006ICJMS..63.1418C. doi:10.1016/j.icesjms.2006.04.019.
- ^ a b c d e f g National Research Council Committee on Effectiveness of Oil Spill Dispersants: Using Oil Dispersants on the Sea, National Academy Press, 1989 pp 63-75
- ^ Tkalich, P Xiaobo, C Accurate Simulation of Oil Slicks, Tropical marine science institute, Presented 2001 International Oil Spill Conference pp 1133-1135 http://www.ioscproceedings.org/doi/pdf/10.7901/2169-3358-2001-2-1133
- ^ a b c d Using Oil Spill Dispersants. National Academy Press. pp 29-32 1989
- ^ a b c d e Butt, Hans-Jürgen. Graf, Karlheinz. Kappl, Michael. "Physics and Chemistry of Interfaces". 2nd Edition. WILEY-VCH. pp 265-299. 2006.
- ^ a b c d e f g Tiehm, Andreas (January 1994). "Degradation of polycyclic aromatic hydrocarbons in the presence of synthetic surfactants". Applied and Environmental Microbiology. 60 (1): 258–263. Bibcode:1994ApEnM..60..258T. doi:10.1128/aem.60.1.258-263.1994. PMC 201297. PMID 8117081.
- ^ a b c d e f Grimberg, S.J.; Nagel, J; Aitken, M.D. (June 1995). "Kinetics of phenanthrene dissolution into water in the presence of nonionic surfactants". Environmental Science & Technology. 29 (6): 1480–1487. Bibcode:1995EnST...29.1480G. doi:10.1021/es00006a008. PMID 22276867.
- ^ a b c d e Egan, Robert; Lehninger, A.; Jones, M.A. (January 26, 1976). "Hydrophile-lipophile balance and critical micelle concentration as key factors influencing surfactant disruption of mitochondrial membranes" (PDF). Journal of Biological Chemistry. 251 (14): 4442–4447. doi:10.1016/S0021-9258(17)33316-1. PMID 932040.
- ^ a b Kim, I.S.; Park, J.S.; Kim, K.W. (2001). "Enhanced biodegradation of polycyclic aromatic hydrocarbons using nonionic surfactants in soil slurry". Applied Geochemistry. 16 (11–12): 1419–1428. Bibcode:2001ApGC...16.1419K. doi:10.1016/S0883-2927(01)00043-9.
- ^ US patent 4992213, G. Troy Mallett, Edward E. Friloux, David I. Foster, "Cleaning composition, oil dispersant and use thereof", published 1991-02-12
- ^ US patent 6261463, Savarimuthu M. Jacob, Robert E. Bergman, Jr., "Water based oil dispersant", published 2001-07-17, assigned to U.S. Polychemical Marine Corp.
- ^ "Study says oil is poison (1974)". The Capital Times. 1974-05-31. p. 50. Retrieved 2020-07-02.
- ^ Mulkins-Phillips, G. J.; Stewart, J.E. (October 1974). "Effect of Four Dispersants on Biodegradation and Growth of Bacteria on Crude Oil". Applied Microbiology. 28 (4): 547–552. doi:10.1128/am.28.4.547-552.1974. PMC 186769. PMID 4418491.
Further reading
- National Academies of Sciences, Engineering, and Medicine (2019). The Use of Dispersants in Marine Oil Spill Response. Washington, DC: The National Academies Press. doi:10.17226/25161. ISBN 978-0-309-47818-2. PMID 32379406. S2CID 133873607.
{{cite book}}: CS1 maint: multiple names: authors list (link)











