
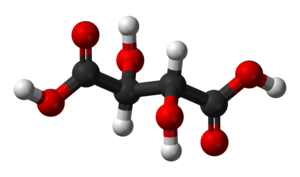
Racemic acid is an old name for an optically inactive or racemic form of tartaric acid. It is an equal mixture of two mirror-image isomers (enantiomers), optically active in opposing directions. Racemic acid does not occur naturally in grape juice, although L-tartaric acid does.
Tartaric acid's sodium-ammonium salt is unusual among racemic mixtures in that during crystallization it can separate out into two kinds of crystals, each composed of one isomer, and whose macroscopic crystalline shapes are mirror images of each other. Thus, Louis Pasteur was able in 1848 to isolate each of the two enantiomers by laboriously separating the two kinds crystals using delicate tweezers and a hand lens.[1] Pasteur announced his intention to resolve racemic acid in:
- Pasteur, Louis (1848) "Sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire"[2]
while he presented his resolution of racemic acid into separate optical isomers in:
- Pasteur, Louis (1850) "Recherches sur les propriétés spécifiques des deux acides qui composent l'acide racémique"[3]
In the latter paper, Pasteur sketches from natural concrete reality chiral polytopes quite possibly for the first time. The optical property of tartaric acid was first observed in 1832 by Jean Baptiste Biot, who observed its ability to rotate polarized light.[4][5] It remains unknown whether Arthur Cayley or Ludwig Schläfli, or other contemporary mathematicians who studied polytopes, knew of the French work.
In two modern-day re-enactments performed in Japan of the Pasteur experiment,[6][7] it was established that the preparation of crystals was not very reproducible. The crystals deformed, but they were large enough to inspect with the naked eye (microscope not required).
See also
References
- ^ George B. Kauffman & Robin Myers (1998). "Pasteur's Resolution of Racemic Acid: A Sesquicentennial Retrospect and a New Translation" (PDF). The Chemical Educator. 3 (6): 1–4. doi:10.1007/s00897980257a. S2CID 95862598. Archived from the original (PDF) on 2006-01-17.
- ^ (On the relations that can exist between crystalline form, chemical composition, and the sense of rotary polarization), Annales de Chimie et de Physique, 3rd series, 24 (3) : 442–459.
- ^ (Investigations into the specific properties of the two acids that compose racemic acid), Annales de Chimie et de Physique, 3rd series, 28 (3) : 56–99. Especially see Plate II. and the report of the commission that was appointed to verify Pasteur's findings, pp. 99–117.
- ^ Biot (1835) "Mémoire sur la polarization circulaire et sur ses applications à la chimie organique" (Memoir on circular polarization and on its applications to organic chemistry), Mémoires de l'Académie des sciences de l'Institut, 2nd series, 13 : 39–175. That tartaric acid (acide tartarique cristallisé) rotates plane-polarized light is shown in Table G following p. 168. (Note: This article was read to the French Royal Academy of Sciences on 1832 November 5.)
- ^ Biot (1838) "Pour discerner les mélanges et les combinaisons chimiques définies ou non définies, qui agissent sur la lumière polarisée; suivies d'applications aux combinaisons de l'acide tartarique avec l'eau, l'alcool et l'esprit de bois" (In order to discern mixtures and chemical combinations, defined or undefined, which act on polarized light; followed by applications to combinations of tartaric acid with water, alcohol [i.e., ethanol], and spirit of wood [i.e., methanol]), Mémoires de l'Académie des sciences de l'Institut, 2nd series, 15 : 93–279.
- ^ Yoshito Tobe (2003). "The reexamination of Pasteur's experiment in Japan" (PDF). Mendeleev Communications Electronic Version. 13 (3): 93–94. doi:10.1070/MC2003v013n03ABEH001803. Archived from the original (PDF) on August 31, 2005.
- ^ Masao Nakazaki (1979). "Morphology of sodium ammonium tartrate: Pasteur's spontaneous resolution and its reexamination". Kagaku No Ryoiki. 33: 951–962.








