| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
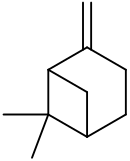
6,6-Dimethyl-2-methylidenebicyclo[3.1.1]heptane
Pin-2(10)-ene | |||
| Other names
6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane
2(10)-Pinene Nopinene Pseudopinene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.004.430 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.872 g/mL | ||
| Melting point | −61.54 °C; −78.77 °F; 211.61 K[1] | ||
| Boiling point | 165–167 °C; 329–332 °F; 438–440 K[2] | ||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−6214.1±2.9 kJ/mol[1] | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H226, H304, H315, H317, H410 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P272, P273, P280, P301+P310, P302+P352, P303+P361+P353, P321, P331, P332+P313, P333+P313, P362, P363, P370+P378, P391, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 36 °C (97 °F; 309 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
β-Pinene is a monoterpene, an organic compound found in plants. It is one of the two isomers of pinene, the other being α-pinene. It is a colorless liquid soluble in alcohol, but not water. It has a woody-green pine-like smell.
β-Pinene is one of the most abundant compounds released by forest trees.[3] If oxidized in air, the allylic products of the pinocarveol and myrtenol family prevail.[4]
Sources
Many plants from many botanical families contain the compound, including:
- Cuminum cyminum[5][6]
- Humulus lupulus[7]
- Pinus pinaster[4]
- Clausena anisata
- Cannabis sativa[8]
- Piper nigrum[9]
- Myristica fragans .[9]
- Citrus aurantiifolia[9]
- Pistacia lentiscus[9]
The clear compound is produced by distillation of turpentine oils.[10]
Uses
This section needs expansion. You can help by adding to it. (October 2023) |
β-Pinene is used in fragrances and essential oils. It is also used in the production of other aroma compounds, such as myrcene and nerol (got by careful fractional distillation of crude nerol got from myrcene[11]). The myrcene is got by pyrolysis of α-Pinene or β-Pinene.[12] Reacting with formaldehyde, result is nopol. When nopol is acetylated, the result is nopyl acetate, which is used as fragrance material.[10][13]
References
- ^ a b "β-Pinene". National Institute of Standards and Technology. Retrieved January 29, 2018.
- ^ "(−)-β-Pinene". Sigma-Aldrich. Retrieved January 29, 2018.
- ^ Geron, C., et al. (2000). A review and synthesis of monoterpene speciation from forests in the United States. Atmospheric Environment 34(11), 1761-81.
- ^ a b Neuenschwander, U.; Meier, E.; Hermans, I. (2011). "Peculiarities of β-pinene autoxidation". ChemSusChem. 4 (11): 1613–21. doi:10.1002/cssc.201100266. PMID 21901836.
- ^ Li, Rong; Jiang, Zi-Tao (2004). "Chemical composition of the essential oil of Cuminum cyminum L. From China". Flavour and Fragrance Journal. 19 (4): 311–313. doi:10.1002/ffj.1302.
- ^ Wang, L.; Wang, Z.; Zhang, H.; Li, X.; Zhang, H. (2009). "Ultrasonic nebulization extraction coupled with headspace single drop microextraction and gas chromatography-mass spectrometry for analysis of the essential oil in Cuminum cyminum L". Analytica Chimica Acta. 647 (1): 72–7. doi:10.1016/j.aca.2009.05.030. PMID 19576388.
- ^ Tinseth, G. The Essential Oil of Hops: Hop Aroma and Flavor in Hops and Beer. Archived 2013-11-11 at the Wayback Machine Brewing Techniques January/February 1994. Accessed July 21, 2010.
- ^ Hillig, Karl W (October 2004). "A chemotaxonomic analysis of terpenoid variation in Cannabis". Biochemical Systematics and Ecology. 32 (10): 875–891. doi:10.1016/j.bse.2004.04.004. ISSN 0305-1978.
- ^ a b c d Santana de Oliveira, Mozaniel (2022). Essential oils: applications and trends in food science and technology. Cham, Switzerland: Springer. ISBN 978-3-030-99476-1.
- ^ a b Surburg, Horst; Panten, Johannes (2016). Common fragrance and flavor materials: preparation, properties and uses (6. completely revised and updated ed.). Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA. ISBN 978-3-527-33160-4.
- ^ Opdyke, D. L. J. (2013-10-22). Monographs on Fragrance Raw Materials: A Collection of Monographs Originally Appearing in Food and Cosmetics Toxicology. Elsevier. ISBN 978-1-4831-4797-0.
- ^ Mattiello, Joseph J. (1945). Protective and Decorative Coatings. U.S. Government Printing Office.
- ^ Opdyke, D. L. J. (2013-10-22). Monographs on Fragrance Raw Materials: A Collection of Monographs Originally Appearing in Food and Cosmetics Toxicology. Elsevier. ISBN 978-1-4831-4797-0.











