 | |
| Clinical data | |
|---|---|
| Trade names | Crestabolic, Cytobolin, Diandren, Madiol, Stenediol, Mestenediol |
| Other names | Metandriol; Methylandrostenediol; Methyl-5-androstenediol; Methylandrostenediole; 17α-Methylandrost-5-ene-3β,17β-diol |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.548 |
| Chemical and physical data | |
| Formula | C20H32O2 |
| Molar mass | 304.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
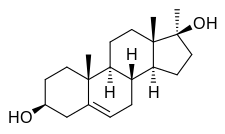
Methandriol (brand names Anabol, Crestabolic, Cytobolin, Diandren, Durabolic, Madiol, Mestenediol, Methabolic, Methydiol, Sterabolic, Stenediol), also known as methylandrostenediol, is an androgen and anabolic steroid (AAS) medication which was developed by Organon and is used in both oral and injectable (as methandriol dipropionate, methandriol propionate, or methandriol bisenanthoyl acetate) formulations.[2][3][4] It is an orally active 17α-alkylated AAS and a derivative of the endogenous androgen prohormone androstenediol.[2][3]
Medical uses
Methandriol has been used in the treatment of breast cancer in women.[5][6][7][8] It has been reported to be almost as virilizing as comparable doses of testosterone propionate and methyltestosterone in women.[9]
Available forms
Methandriol (brand name Androteston M, Notandron, Protandren) was previously marketed as 25 mL and 50 mg/mL aqueous suspensions for use by intramuscular injection.[10]
Chemistry
Methandriol, also known as 17α-methyl-5-androstenediol or as 17α-methylandrost-5-ene-3β,17β-diol, is a synthetic androstane steroid and a 17α-alkylated derivative of 5-androstenediol (androst-5-ene-3β,17β-diol).[2][3] A number of esters of methandriol exist, including methandriol dipropionate (methylandrostenediol 3β,17β-dipropionate), methandriol propionate (methylandrostenediol 3β-propionate), methandriol bisenanthoyl acetate (methylandrostenediol 3β,17β-dioxononanoate), and methandriol diacetate (methylandrostenediol 3β,17β-diacetate; never marketed).[2][3] Methandriol is closely related to methyltestosterone (17α-methyltestosterone or 17α-methylandrost-4-ene-17β-ol-3-one).[2][3]
An analogue of methandriol is its positional isomer methyl-4-androstenediol (17α-methylandrost-4-ene-3β,17β-diol).[11] Another analogue of methandriol is ethynylandrostanediol (17α-ethynyl-5α-androstane-3β,17β-diol) as well as its ester ethandrostate (ethynylandrostanediol 3β-cyclohexylpropionate).[11]
History
Methandriol was first synthesized in 1935 along with methyltestosterone and mestanolone.[5][12][13]
| Route | Medication | Form | Dosage | |
|---|---|---|---|---|
| Oral | Methyltestosterone | Tablet | 30–200 mg/day | |
| Fluoxymesterone | Tablet | 10–40 mg 3x/day | ||
| Calusterone | Tablet | 40–80 mg 4x/day | ||
| Normethandrone | Tablet | 40 mg/day | ||
| Buccal | Methyltestosterone | Tablet | 25–100 mg/day | |
| Injection (IM or SC) | Testosterone propionate | Oil solution | 50–100 mg 3x/week | |
| Testosterone enanthate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Testosterone cypionate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Mixed testosterone esters | Oil solution | 250 mg 1x/week | ||
| Methandriol | Aqueous suspension | 100 mg 3x/week | ||
| Androstanolone (DHT) | Aqueous suspension | 300 mg 3x/week | ||
| Drostanolone propionate | Oil solution | 100 mg 1–3x/week | ||
| Metenolone enanthate | Oil solution | 400 mg 3x/week | ||
| Nandrolone decanoate | Oil solution | 50–100 mg 1x/1–3 weeks | ||
| Nandrolone phenylpropionate | Oil solution | 50–100 mg/week | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | ||||
Society and culture
Generic names
Methandriol is the generic name of methylandrostenediol and its INN.[2][3]
Availability
Methandriol remains marketed for clinical use only in Taiwan and for veterinary use (as methandriol dipropionate) only in Australia.[14]
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-15.
- ^ a b c d e f Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 794–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 177–. ISBN 978-94-011-4439-1.
- ^ Thomas JA, Keenan EJ (6 December 2012). Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 125–. ISBN 978-1-4684-5036-1.
- ^ a b Henderson E, Weinberg M (June 1951). "Methylandrostenediol". The Journal of Clinical Endocrinology and Metabolism. 11 (6): 641–652. doi:10.1210/jcem-11-6-641. PMID 14841252.
- ^ Homburger F, Kasdon SC, Fishman WH (May 1950). "Methylandrostenediol: a non-virilizing derivative of testosterone in metastatic cancer of the breast". Proceedings of the Society for Experimental Biology and Medicine. 74 (1): 162–164. doi:10.3181/00379727-74-17840. PMID 15430420. S2CID 209361921.
- ^ Kasdon SC, Fishman WH, Dart RM, Bonner CD, Homburger F (April 1952). "Methylandrostenediol in palliative treatment of breast cancer". Journal of the American Medical Association. 148 (14): 1212–1216. doi:10.1001/jama.1952.02930140044014. PMID 14907362.
- ^ Segaloff A, Gordon D, Horwitt BN, Schlosser JV, Murison PJ (March 1952). "Hormonal therapy in cancer of the breast. II. Effect of methylandrostenediol on clinical course and hormonal excretion". Cancer. 5 (2): 271–274. doi:10.1002/1097-0142(195203)5:2<271::AID-CNCR2820050212>3.0.CO;2-W. PMID 14905410. S2CID 39681958.
- ^ Harold Gardiner-Hill (1958). Modern Trends in Endocrinology. Butterworth. p. 235.
Foss (1956), using methylandrostenediol in doses of 100 milligrams daily in the treatment of patients with inoperable carcinoma of the breast, found it almost as virilizing as testosterone propionate or methyltestosterone in comparable doses.
- ^ Heinrich Kahr (8 March 2013). Konservative Therapie der Frauenkrankheiten: Anzeigen, Grenzen und Methoden Einschliesslich der Rezeptur. Springer-Verlag. pp. 21–. ISBN 978-3-7091-5694-0.
- ^ a b Bernstein S, Stolar S, Heller M (1957). "Notes - Synthesis of 17α-Methyl-4-androstene-3β,17,β-diol". The Journal of Organic Chemistry. 22 (4): 472–473. doi:10.1021/jo01355a626. ISSN 0022-3263.
- ^ Schänzer W (July 1996). "Metabolism of anabolic androgenic steroids". Clinical Chemistry. 42 (7): 1001–1020. doi:10.1093/clinchem/42.7.1001. PMID 8674183.
- ^ Ruzicka L, Goldberg MW, Rosenberg HR (1935). "Sexualhormone X. Herstellung des 17-Methyl-testosterons und anderer Androsten- und Androstanderivate. Zusammenhänge zwischen chemischer Konstitution und männlicher Hormonwirkung". Helvetica Chimica Acta. 18 (1): 1487–1498. doi:10.1002/hlca.193501801203. ISSN 0018-019X.
- ^ "List of Androgens and anabolic steroids". Drugs.com.








