| |
| Names | |
|---|---|
| IUPAC name
Sodium bis(2-methoxyethoxy)aluminium hydride
| |
| Other names
Red-Al, Synhydrid, Vitride
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | SMEAH |
| ChemSpider | |
| ECHA InfoCard | 100.041.056 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H16AlNaO4 | |
| Molar mass | 202.161 g·mol−1 |
| Appearance | Transparent crystals |
| Density | 1.122 g/cm3 (solid)[1] 1.036 g/mL (>60% solution) |
| Viscosity | 65.1 cps (70% solution)[citation needed] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly flammable; moisture sensitive; potent skin irritant[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
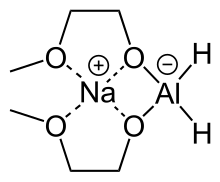
Sodium bis(2-methoxyethoxy)aluminium hydride (SMEAH;[1] trade names Red-Al, Synhydrid, Vitride) is a hydride reductant with the formula NaAlH2(OCH2CH2OCH3)2. The trade name Red-Al refers to its being a reducing aluminium compound. It is used predominantly as a reducing agent in organic synthesis. The compound features a tetrahedral aluminium center attached to two hydride and two alkoxide groups, the latter derived from 2-methoxyethanol. Commercial solutions are colorless/pale yellow and viscous. At low temperatures (below -60°C), the solution solidifies to a glassy pulverizable substance with no sharp melting point.
SMEAH is a versatile hydride reducing agent. It readily converts epoxides, aldehydes, ketones, carboxylic acids, esters, acyl halides, and anhydrides to the corresponding alcohols. Nitrogen derivates such as amides, nitriles, imines, and most other organonitrogen compounds are reduced to the corresponding amines. Nitroarenes can be converted to azoxyarenes, azoarenes, or hydroazoarenes, depending on the reaction conditions.[1]
Some common functional group reductions using SMEAH can be found below:

Comparison with lithium aluminium hydride
As a reagent, SMEAH is comparable with lithium aluminium hydride (LAH, LiAlH4).
It is a safer alternative to LAH and related hydrides. SMEAH exhibits similar reducing effects, but does not have the inconvenient pyrophoric nature, short shelf-life, or limited solubility of LAH. Upon contact with air and moisture, SMEAH reacts exothermically but does not ignite, and tolerates temperatures up to 200°C. Under dry conditions it has unlimited shelf life. It is soluble in aromatic solvents, whereas LAH is only soluble in ethers. For example, a solution greater than 70 wt.% concentration in toluene is commercially available. The reagent can be modified to effect partial reductions.[1]
SMEAH in toluene under reflux has been used to reduce aliphatic p-toluenesulfonamides (TsNR2) to the corresponding free amines and is one of the few reagents that can carry out this challenging reduction in general settings. Notably, LiAlH4 does not reduce this functional group unless forcing conditions are used.[2]
References
- ^ a b c d e Gugelchuk, M.; Silva, L. F. III; Vasconcelos, R. S.; Quintiliano, S. A. P. (2007). "Sodium Bis(2-methoxyethoxy)aluminum Hydride". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/9780470842898.rs049.pub2. ISBN 978-0471936237.
- ^ Smith, Michael B. (2011). Organic Synthesis. Cambridge, Mass.: Academic Press. p. 368. ISBN 9780124158849.
External links
- "Red-Al, Sodium bis(2-methoxyethoxy)aluminumhydride". Organic Chemistry.








